As students across the country prepare to return to in-person learning this fall, schools are closely watching federal, state and local health guidelines—and listening to parents and staff about what feels safe and right when kids return to campus.
Since children under 12 years of age are not yet eligible for vaccination, national public health experts have recognized the value of routine rapid response coronavirus testing for elementary and middle school settings in particular. One approach is to conduct rapid antigen testing.
Routine testing gives teachers, parents, and students peace of mind that they are operating in a safe learning environment. That’s why the federal government committed over $12 billion toward building up COVID-19 testing in schools. Now is the time for school districts to consider the details of implementing a testing program to ensure a smooth and safe return to classrooms this fall.
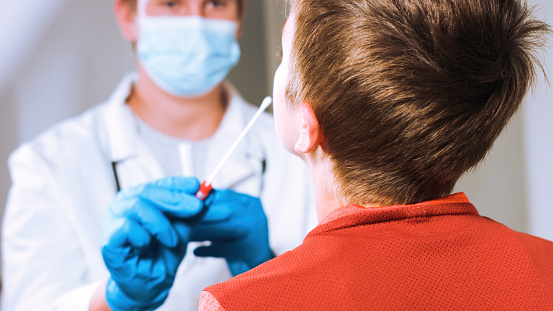
Antigen tests are fast and simple to use with some tests producing results in as little as 15 minutes. These tests make COVID-19 easy to identify, track and, if necessary, contain. In fact, elementary schools, athletic teams and other educational settings around the U.S. are already using rapid antigen testing to keep students, teachers and staff safe.
While rising vaccine rates and falling caseloads are good signs that we may soon be able to put this pandemic behind us, we cannot become complacent. Outbreaks continue to occur around the world, and we must remain vigilant to keep our schools and communities open and safe. To be fully prepared for the start of school, administrators should be developing testing programs now, ideally using both polymerase chain reaction (PCR) tests and rapid antigen tests.
Kevin Kelly serves as senior vice president/general manager for BD’s Point of Care business, which developed the BD Veritor™ Plus System. This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner. For more information, please see bdveritor.com.





